The First Principal Energy Level Is Called the
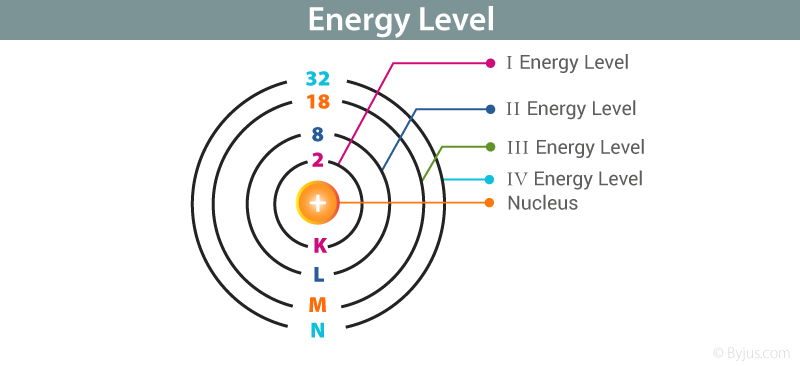
The first electronic shell or energy level having n 1 called the K shell is the closest to the nucleus. An S-Orbital in the first energy level is a 1s orbital.
Question text The first principle energy level is called the.

. Called an electron configuration. Where R H is called Rydberg constant whose value is 21810 18 J. New questions in Chemistry The first principle energy level is called The best method for studying is cramming the night before true or false Which TWO statements describe the seismic wave known as an S-wave.
The highest-energy principal energy level containing electrons is the third energy level and that energy level contains one electron. The first energy level is also called level K. Electron configurations can make it easy to see the valence shells for the atoms of the elements.
The orbits have quantized sizes and energies. The second level is called level L third energy level as M and so on. The value o n is sometimes called the principle auantum number.
GENERAL CHEMISTRY 1 LAB EXAM 5 The first principle energy level is called the. How many electrons are in each energy level1 point 1two in the first energy level six in the second energy level 2eight in the first. First let us point out those features using the complete periodic table shown in Figure 510.
History The first evidence of quantization in atoms was the observation of spectral lines in light from the sun in the early 1800s by Joseph von Fraunhofer and William Hyde Wollaston. The first principle energy level is called the. None of the known elements in its ground state has any electrons in a principal energy level.
True The first quantum number identifies the principal energy level of an electron. Calculate the energy of the electron in the ground state of single ionized helium which. Experts are tested by Chegg as specialists in their subject area.
Measurement of the possible energy levels of an object is called spectroscopy. Up to 24 cash back Every energy level contains one S-Orbital. This preview shows page 1 - 2 out of 4 pages.
The 1 represents the first principal energy level the s indicates an electron cloud with a spherical shape and the 2 shows that there are two electrons in that 1s sublevel. 1What is the longest wavelength light capable of ionizing a hydrogen atom in the n 6 state 2a. Hunds state Feedback The correct answer is.
Each shell has a different energy level increasing the further it is from the nucleus. 1s2 Represents the principal energy level Indicates the shape of the orbital Shows the number of electrons in the orbital. True An electron can behave as either a wave or a particle.
View Quiz4docx from CHM MISC at University of Florida. True No two electrons in the same atom can have the same set of four quantum numbers. The Pirst energy level can only hold 2- electrons the second can only hold 8 and the third can only hold 18.
The next shell has a value of n2 etc. In an atom the sub atomic particles called electrons revolve around the nucleus of the given atom at different energy levels called shells. Hydrogen H and helium He have a valence shell containing one.
Who are the experts. The electrons are arranged in highest or main energy level that consist of one or more sublevels. This level is denoted by the principal quantum number n.
Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. There are shaped like a 3D figure of eight. This level is denoted by the principal quantum number n.
Yan po ang answer. The energy of an electron when it is far away from the influence of the nucleus is taken as zero. The general region of space where an electron is most likely to be found is known as an orbital.
And the energy level they occupy. In chemistry the principal energy level of an electron refers to the shell or orbital in which the electron is located relative to the atoms nucleus. We review their content and use your feedback to.
The electrons from energy level K contains the least energy whereas the levels that are far from the nucleus contains more energy Electrons in the outermost energy level are also called Valence electrons. College The first principle energy level is called sylviaruthwilson1971 is waiting for your help. For a neutral element this energy is a measure of.
Principle one state b. The way in which electrons are distributed in the different orbitals around the nucleus of an atom is called the principal. If n is the number associated with the principal energy level each principal energy level has n sublevels.
In chemistry the principal energy level of an electron refers to the shell or orbital in which the electron is located relative to the atoms nucleus. -for any principal energy level n the total number of orbitals possible for that level is equal to n2. 12 out of 14 people found this document helpful.
The closest shell has a value of n1. There are no full energy levels in an atom of. Add your answer and earn points.
2Page Each energy level can only hold a certain number o-P electrons. An energy level is the fixed amount of energy that a system described by quantum mechanics such as a molecule atom electron or nucleus can have. The first element in a period of the.
They exist in groups of three. The first element in a period of the. Nucleus 1st shell 2 electrons 2nd shell B electrons d shell 18 electrons.
Every energy level except the first level contains three P-Orbitals. Each energy level is given a number called the principal quantum number n. The maximum number of electrons possible in the first four energy levels are.
Filling of electrons start from lowest energy level to the electron energy level. Shown here is the first Balmer transition in which an electron jumps from orbit n 3 to orbit n 2 producing a photon of red light with an energy of 189 eV and a wavelength of 656 nanometres. They are often together called the valence shell.
Principal quantum number of an electron existing in such a stationary state is taken as n. Orbitals of _____ energy are filled first. In the table the elements are placed in rows and columns of varying length.
An S-Orbital in the second energy level is a 2s orbital etc. The valence electrons for the representative main group of elements are found in the outermost highest energy s and p sublevels. Note that the first principal energy level has one sublevel the second has two the third has three and the fourth has four.
The energy required for the complete removal of 1 mol of electrons from 1 mol of gaseous atoms or ions is called _____ energy.
How Many Electrons Can The 4th Energy Level Hold At Level
Energy Level Principal Quantum Number Bohr S Atomic Model Physics

Condensed Electron Configuration Valence And Energy Diagrams Mike S Videos Dat Bootcamp Electron Configuration Teaching Chemistry High School Chemistry
No comments for "The First Principal Energy Level Is Called the"
Post a Comment